Episode 3: The Risky Business of CVD Risk Assessment
In the third session, we discuss the advantages and disadvantages of three methods to present “benefit”: changes in risk calculators, using relative risk, or the absolute benefit. We review the challenges of absolute vs relative risk (or relative vs absolute truth) and discuss patient expectations in regards to the medical miracle of prevention. The duration of therapy is put in context of the epoch time frames of risk calculators and studies.
Show Notes
Definitions
CVD is cardiovascular disease and typically refers to the combination of CHD (coronary heart disease – fatal and non-fatal MIs and sometimes angina) PLUS cerebrovascular disease (fatal and non-fatal strokes – and sometimes TIAs) PLUS (sometimes) other conditions (heart failure, peripheral vascular disease)
Calculating benefit
- Change the factor and recalculate the chance of CVD
- Use the relative benefits seen in clinical trials (typically 5 years in duration) and apply them to the chance calculated for your patient
- Avoid the use of CDV calculators and just use the absolute benefits seen in clinical trials
A synopsis of the relative benefit of drugs
- Statins ? 30%? in CHD (0%? in women)? 5 years
- BP ? 40 %? in strokes and ? 20%? in CHD ? 5 years
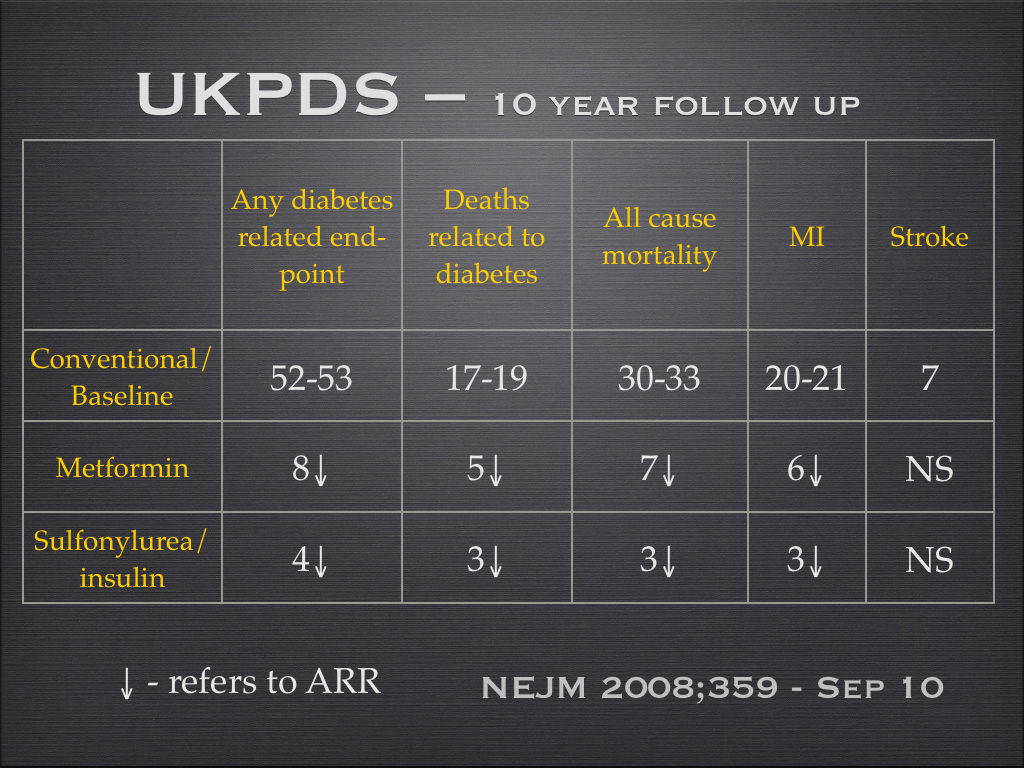
- Metformin ? 35%? in CHD and stroke ? 8-10 years
A synopsis of the absolute benefit of drugs
Statins over 5 years in a post MI patient Coronary events ?4% (15% to 11%) Death ?2% (12% to 10%) Strokes ?1% (5% to 4%) Treating a Blood Pressure of 160 /100 mmHg for 5 years CVD ? 1% (4% to 3%)
Absolute benefit of statins over approx 5 years
| Major coronary events (%)* | Death (%) | Strokes (%) | FROM WHAT CVD TO WHAT CVD (%) | |
| Primary | 1-1.5* | – | – | 8-9 to 7 |
| Diabetes | 2 | – | 1-1.5 | 10 to 7 |
| Secondary | 4 | 2 | 1 | 20 to 15 |
* just in males and NO difference in overall serious adverse events